
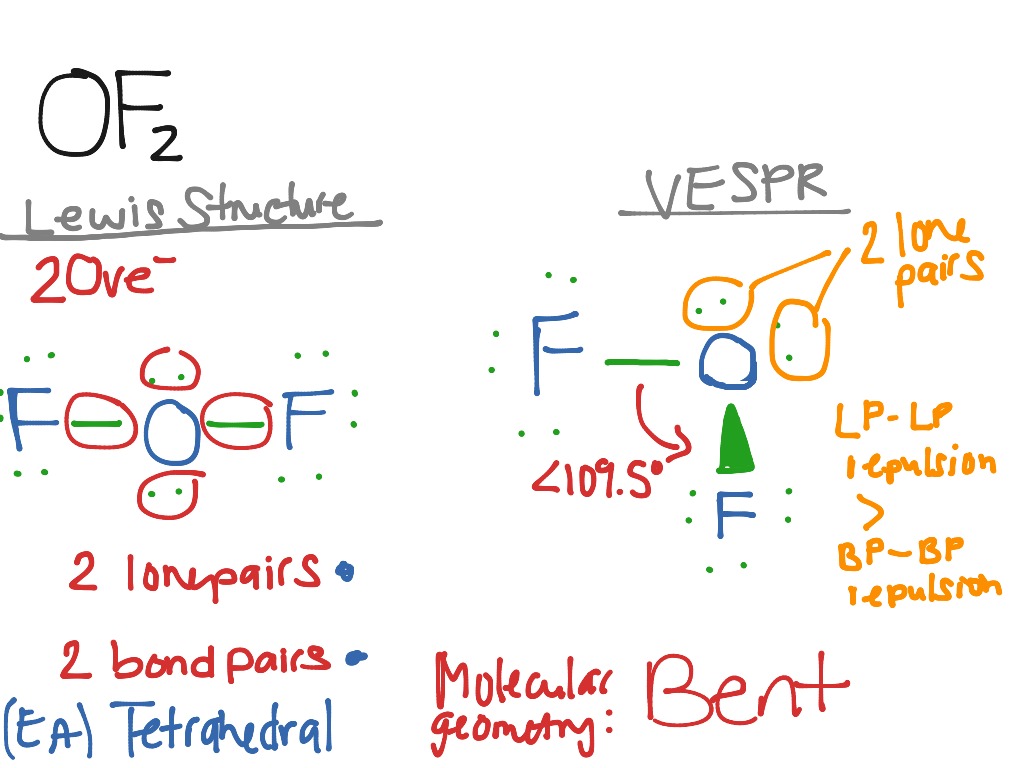
For molecules of water and ammonia, however, the non-bonding electrons must be included in the calculation. In the three examples shown above, the central atom (carbon) does not have any non-bonding valence electrons consequently the configuration may be estimated from the number of bonding partners alone. The bonding configurations of carbon are easy to remember, since there are only three categories. This simple model is based on the fact that electrons repel each other, and that it is reasonable to expect that the bonds and non-bonding valence electron pairs associated with a given atom will prefer to be as far apart as possible. īonding configurations are readily predicted by valence-shell electron-pair repulsion theory, commonly referred to as VSEPR in most introductory chemistry texts. The following examples make use of this notation, and also illustrate the importance of including non-bonding valence shell electron pairs (colored blue) when viewing such configurations. a covalent bond that is partially formed or partially broken). Some texts and other sources may use a dashed bond in the same manner as we have defined the hatched bond, but this can be confusing because the dashed bond is often used to represent a partial bond (i.e. A wedge shaped bond is directed in front of this plane (thick end toward the viewer), as shown by the bond to substituent B and a hatched bond is directed in back of the plane (away from the viewer), as shown by the bond to substituent D. The two bonds to substituents A in the structure on the left are of this kind. As defined in the diagram on the right, a simple straight line represents a bond lying approximately in the surface plane. In most cases the focus of configuration is a carbon atom so the lines specifying bond directions will originate there. In order to represent such configurations on a two-dimensional surface (paper, blackboard or screen), we often use perspective drawings in which the direction of a bond is specified by the line connecting the bonded atoms. This shape is dependent on the preferred spatial orientation of covalent bonds to atoms having two or more bonding partners.Three dimensional configurations are best viewed with the aid of models.
Molecular dipole moment definition series#
The denominators of these equation can be expanded using the multipole expansion to give a series of terms that have larger of power of distances in the denominator.The three dimensional shape or configuration of a molecule is an important characteristic. M = − x ^ ∂ U int ∂ B x − y ^ ∂ U int ∂ B y − z ^ ∂ U int ∂ B z. The magnetic moment can be defined as a vector relating the aligning torque on the object from an externally applied magnetic field to the field vector itself. The dipole component of an object's magnetic field is symmetric about the direction of its magnetic dipole moment, and decreases as the inverse cube of the distance from the object.ĭefinition, units, and measurement Definition The magnetic field of a magnetic dipole is proportional to its magnetic dipole moment.

The direction of the magnetic moment points from the south to north pole of the magnet (inside the magnet). The magnetic moment may be considered, therefore, to be a vector. The strength (and direction) of this torque depends not only on the magnitude of the magnetic moment but also on its orientation relative to the direction of the magnetic field. The same applied magnetic field creates larger torques on objects with larger magnetic moments. The magnetic dipole moment of an object is readily defined in terms of the torque that the object experiences in a given magnetic field. Higher-order terms (such as the magnetic quadrupole moment) may be needed in addition to the dipole moment for extended objects. The magnetic dipole component is sufficient for small enough magnets or for large enough distances.

More precisely, the term magnetic moment normally refers to a system's magnetic dipole moment, the component of the magnetic moment that can be represented by an equivalent magnetic dipole: a magnetic north and south pole separated by a very small distance. Examples of objects that have magnetic moments include loops of electric current (such as electromagnets), permanent magnets, elementary particles (such as electrons), various molecules, and many astronomical objects (such as many planets, some moons, stars, etc). In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field.


 0 kommentar(er)
0 kommentar(er)
